Services
Language, IT, Design, Specialist Categories, Research, and Education:
Six areas of expertise that SunFlare's human resources leverage to facilitate global communication
SunFlare pioneers research in document sciences, which involves resolving the diverse range of document-related challenges that our clients face in today's global business environment. We call this solution Document Soken. We provide our clients with the support they need to succeed as a global business by combining the expertise and knowledge that our human resources possess in the following six areas: Language (our core competency), IT, Design, Research, Education, and Specialist Categories.
Service categories
-
 Translation
TranslationWith a diverse lineup of field-specific experts, we can handle translations in over 90 languages.
-
 Writing
WritingWe can produce a wide range of highly specialized documentation, from product manuals to medical documents.
-
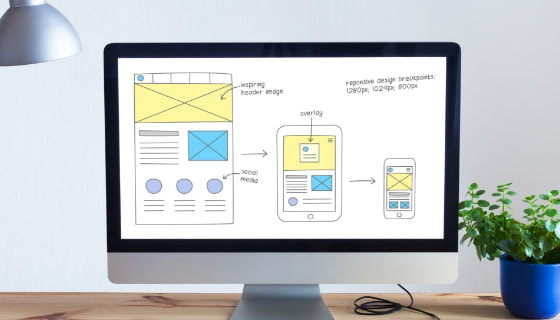 Creative Services
Creative ServicesWe can optimize your document output. We produce documents in a variety of different media, including print and online.
-
 Research and Consultation
Research and ConsultationWith a talent pool of around 6,000 people, we are able to offer a comprehensive range of research and document consultation services.
-
 Training and Seminars
Training and SeminarsWe deliver a diverse range of seminars and corporate language training programs.
Industries and business categories
We cover all industries, fields and sectors
SunFlare has worked for over half a century in business translation for leading-edge science and technology as well as many other industries.
We leverage our knowledge backed by an extensive track record, experience and powerful foresight to provide solutions in translation, production and much more. Our services bring true value to our clients in all industries, fields and sectors.

Food, marine products, agriculture and forestry
Food, Marine products, Fishery, Agriculture, Forestry

Trading, retail, distribution and food service
General trading, Specialized trading, Wholesale, Pharmaceutical wholesale, Retail, Mail-order and e-commerce, Apparel, Food service

Resources and energy
Mining, Petroleum, Coal, Electric power, Gas, Nuclear power generation, Nuclear fusion, Wind power generation, Geothermal power generation, ILC, Energy
Finance and insurance
Banking, Securities, Insurance, Investment funds, Leasing

Materials and chemistry
Fibers, Pulp, Chemistry, Glass, Rubber, Metals, Steel, Non-ferrous metals

Transportation and logistics
Warehousing, Logistics, Land transportation, Ocean transportation, Air transportation
Machinery and electronics
Machinery, Electrical devices and electronics, Semiconductors, Semiconductor manufacturing equipment, Precision instruments, Medical devices, Robots, Space development, IoT, Quantum computing

Telecommunications, Internet and media
Systems, Software, IT services, Communications, Broadcasting, Newspapers, Publishing, Advertising, Printing

Means of transportation
Automobiles, Motorcycles, Bicycles, Electric vehicles and next-generation transportation, Aircraft, Railway, Shipbuilding, Space development

Hobbies and entertainment
Leisure, Travel, Hotels, Sports, eSports, Music, Movies, Anime, Videogames, Casinos and resorts
Life science
Pharmaceuticals, Biotechnology, Medical devices, Regenerative medicine, Agrochemicals, Animal medicine, Diagnostic pharmaceuticals, Drug discovery ventures, CRO, SMO, Medicine, Pharmaceutical wholesale, Food, Cosmetics

Specialist and corporate services
Attorneys, Patent attorneys, Certified public accountants, Tax accountants, Real estate appraisers, Judicial scriveners, Administrative scriveners, Audit corporations, Consultancies, Think tanks, Human resources services, Design

Household goods and luxuries
Toiletries, Stationery and office supplies, Toys, Clocks and watches, Glasses, Shoes, Clothing, Jewelry, Cigarettes

Scientific research and education
Educational institutions, Research institutions, Academic societies, Universities, Graduate schools, Technical colleges, Super Global High Schools, Preparatory schools

Civil engineering, construction and real estate
Civil engineering, Erosion control, Harbors, Construction, Architecture, Housing, Residential facilities, Real estate

Life and public affairs
Government and municipal offices, Independent administrative agencies, Nonprofit foundations, Medicine, Nursing care, Welfare, Environment, Ceremonial occasions, Security, Defense
Top-class transaction results in the translation industry
SunFlare provide comprehensive documentation services that cater to clients in all business divisions and occupations
Each corporate division faces a broad range of challenges as it strives to succeed in global communication and business.
At SunFlare, we understand the fundamental essence of these challenges and we possess the know-how to solve them while offering coverage for highly specialized fields that we have cultivated over many years.
- Annual number of transactions:
- 29,544 cases
- Annual number of clients:
- 1,388 companies
Languages
SunFlare caters to a wide range of translation needs for customers in any language
We work in over 90 languages from all around the world, including America, Europe, the Middle East, Africa, Asia and Oceania.
Our services cover both official languages and local languages, allowing for precise and accurate global solutions.
- We provide translation services for
more than - 90 languages
Languages (translations available in both directions)
America
North America
- French
- English
South America
- Dutch
- Spanish
- Brazilian Portuguese
- French
- English
Europe
Eastern Europe / Russia
- Azerbaijani
- Ukrainian
- Greek
- Georgian
- Slovak
- Czech
- Turkish
- Hungarian
- Bulgarian
- Belarusian
- Polish
- Romanian
- Russian
Western Europe
- Irish
- Italian
- Dutch
- German
- French
- Luxembourgish
- Romansch
Southern Europe
- Albanian
- Italian
- Catalan
- Greek
- Croatian
- Spanish (EU)
- Slovenian
- Serbian
- Bosnian
- Portuguese (EU)
- Macedonian
- Maltese
- English
Northern Europe
- Icelandic
- Estonian
- Swedish
- Danish
- Norwegian
- Finnish
- Latvian
- Lithuanian
- English
Middle East and Africa
Middle East and Africa
- Amharic
- Arabic
- Spanish
- Swahili
- French
- Hebrew
- Portuguese
- English
Asia and Oceania
East Asia
- Mongolian
- English
- Korean
- Chinese (Cantonese)
- Chinese (Taiwanese)
- Chinese (Mandarin)
South Asia
- Urdu
- Sinhalese
- Dzongkha
- Tamil
- Dari
- Nepali
- Pashto
- Punjabi
- Hindi
- Persian
- Bengali
- Marathi
- Malayalam
- English
South East Asia
- Indonesian
- Cambodian
- Khmer
- Javanese
- Thai
- Tagalog
- Tamil
- Tetum
- Vietnamese
- Portuguese
- Malay
- Burmese
- Lao
- English
- Chinese (Cantonese)
Central Asia
- Uzbek
- Kazakh
- Kyrgyz
- Tajik
- Turkmen
- Russian
Oceania
- Greek
- Hawaiian
- Hindi
- French
- English



